|
Given the
exponential form of the decay, the sample can take a long time to
become inactive. This, together with the fact that the decay processes
cannot be accelerated, is one of the dangers of radioactivity.
|
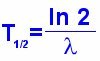
If we have a million
radioactive atoms and the half life is one year, they will take a year
to decrease to 500.000.
But at the end of this year there will still be 250.000, and
another year later 125.000. Less and less atoms decay in the same
time period.
No of initial atoms T1/2
to become No /2.
|
