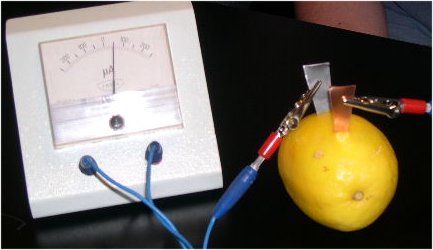
You can make your own battery with a lemon, a piece of copper and a piece of zinc.
It produces very few electrons (microamperes) and they are unfortunately not enough to turn on a light bulb.
However, if you accumulate the electrons in a capacitor you can turn a light bulb on in the discharge of the condenser.
|
The greater the size of the zinc and copper plates stuck in the lemon, the greater the surface that is attacked by the acid, which means that more electrons are produced and the intensity is greater.
The voltage produced by the battery depends on the materials and it does not change if the plates are larger.
When the plates are Zn and Cu the voltage is 1 volt.
|
